IIT Hyderabad Researchers ready for transfer of technology to treat ‘Black Fungus’
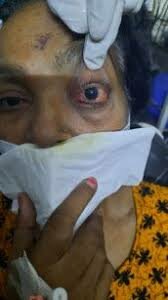
Hyderabad: In order to treat Black Fungus (Mucormycosis) and other fungi and provide relief to patients, the researchers at the Indian Institute of Technology-Hyderabad (IIT-H) have developed an oral solution. IIT-H had developed nano-fibre based, controlled release oral tablets of amphotericin B (AmB) to treat the fungal infections.
For mass-scale production, the researchers are confident that the technology can be transferred to suitable pharma partners to take it forward. To ensure the availability and affordability of the drug, the technology developed is made free from Intellectual Property Rights.
To ensure easy medicare for patients, a 60-mg tablet is priced at a cost of Rs 200 only. The tablet is said to be patient friendly as the drug’s slow and sustained release in the body reduces nephrotoxicity, that is, ill impact of medicines and chemicals on the kidney.
IIT-H has stated that the research is being funded by DST-Nanomission, intended to deliver AmB orally at a slow rate within the therapeutic window aggregation in order to lower drug toxicity. To satisfy this phenomenon, the team has selected gelatin, an FDA approved polymer as an excipient for drug molecules.
Amphotericin-B is currently manufactured in the form of an injection by a few pharmaceutical companies. Its use in the treatment of black fungus costs lakhs of rupees; a 50 mg vial costs close to Rs 4,000, and up to 60-100 vials are required for one patient.
Mucormycosis is listed to be a very rare infection which is caused by exposure to mucor mound which is commonly found in soil, plants, manure, and decaying fruits and vegetables. It can be life-threatening and affects the sinuses, the brain and the lungs. Doctors believe that the fungal infection which has an overall mortality rate of 50%, may be triggered by the use of steroids, a life-saving treatment for severe and critically ill Covid-19 patients.